Over 30 years after Dow AgroSciences launched Starane, a brand new active from the same chemical group promises to broaden the window and spectrum of reliable broadleaf weed control. CPM follows the story.
From the moment the greenhouse test results started coming in, we knew we had something that was of great interest.
By Tom Allen-Stevens
There’s a group of broadleaf weed herbicides that may, perhaps unfairly, have a reputation for being outdated and somewhat clunky. The synthetic auxins include the likes of MCPA, mecoprop and 2,4-D – chemicals you probably keep a can or two of, but they’re rarely the first line of defence.
There’s one in that same group, however, that’s so dependable for its efficacy on cleavers it’s become a household name (on most arable farms, at least). Starane (fluroxypyr) was launched in the mid 1980s and remains the product of choice for cleaver control, right up to GS45.
Its manufacturer, Dow AgroSciences, has now launched Arylex (halauxifen-methyl) – another synthetic auxin, although in a new class of chemistry. Both actives have been combined in Pixxaro, that was available to UK growers for the first time this spring. So does the new arrival live up to and indeed complement its sister molecule?
Godsend for cleavers
“I can remember when Starane was introduced, and it was a godsend for cleaver control,” comments John Youles of TAG Consulting. “At the time all we had to control them in the spring was CMPP, which needed warm conditions and you couldn’t it use past GS31. But as growers were drilling wheat earlier in the autumn, by the time temperatures warmed up in the spring, cleavers were hardening and were as big as dinner plates.
“So Starane really was revolutionary – it has an application window up to ear emergence and will reliably control cleavers that are clambering all over your crop.”
Anne Thompson, Dow AgroSciences UK R&D and regulatory leader, worked on the development of Starane for the UK market. “Two things worked in tandem to propel Starane to widespread use: farmers were becoming more aware

Once Starane was launched, its uptake was phenomenal, recalls Anne Thompson
of how big a problem cleavers were and the economic losses that could result, while none of the products available at the time offered reliable control.”
Cleavers have the reputation as the most competitive arable weed – data from NIAB TAG suggest yield loss from a single seedling is more than seven times that of blackgrass. So in 1976, when Dow chemists in Walnut Creek, California, noted triclopyr, a pasture herbicide, had activity on the weed, it prompted a re-evaluation of the entire series of related chemistry.
Three new compounds were subsequently evaluated at Dow’s agricultural research station in Kings Lynn, Norfolk, recalls Anne Thompson, and they singled out one that showed true promise. “Fluroxypyr came out on top – it had the best broadleaf activity and cereal selectivity. It was a different level of control to what we’d seen before, and worked on quite large cleavers. The only thing it didn’t do well was control them in cold weather.”
Extremely effective
Trials carried out by Dow and ADAS in the UK showed the new active was extremely effective, and it received its approval for use in Nov 1984. “Its uptake was phenomenal. In its first year of use, market research shows it was used on 11% of the market for straight active ingredients used as phenoxy herbicides and achieved 11% market penetration co-formulated in broad-spectrum products. Starane was applied to 5% of all cereals grown in 1985,” she notes.
Growers quickly worked out how best to use it, recalls John Youles. “It needs warm conditions but it’s always highly effective. Dow then rejuvenated the product, adding florasulam, which was marketed as Starane XL, and that brought activity at lower temperatures.”
But fluroxypyr has a limited spectrum of activity, he points out. “Apart from cleavers, and some very valuable control of volunteer potatoes, there are not many broadleaf weeds that Starane controls. The rise of the sulfonylureas (SU) during the 1990s really took spring broadleaf weed control to the next level. Starane and Ally (metsulfuron-methyl) went together like bread and jam.”
Unlike synthetic auxins, however, SUs are susceptible to resistance. “It’s a rising problem – we’ve seen it in poppies, chickweed and to a more limited degree in mayweed. I have a case near Bourne in Lincs of poor control using SUs. We’ve now switched back to MCPA and florasulam, and rely more on pendimethalin,” notes John Youles.
Meanwhile the chemists at Dow in Indianapolis, USA, had been pursuing a new group of compounds. “It’s a chemical family known as the arylpicolinates,” explains lead research specialist Paul Schmitzer.
Pixxaro weed spectrum
| Weed | 0.375 l/ha + adjuvant | 0.5 l/ha + adjuvant |
| Black bindweed | S<10cm | S<15cm |
| Black nightshade | – | S<4 leaf |
| Charlock | T | T |
| Chickweed | S<25cm | S<flowering |
| Cleavers | before flowering | before flowering |
| Clover | – | S<4 leaf |
| Cranesbill | S<10cm | S<20cm |
| Docks | – | S<4 leaf |
| Fat hen | S<15cm | S<25cm |
| Forget-me-not | MS<10cm | S<10cm |
| Fumitory | S<20cm | before flowering |
| Groundsel | T | MS |
| Hemp-nettle | S< 10cm | before flowering |
| Henbit deadnettle | S<flowering | S<flowering |
| Knotgrass | MS<10cm | S< 8cm |
| Marigold | T | T |
| Mayweed | T | T |
| Nettle, small | S | S |
| Orache | MS | MS |
| Pale persicaria | MS | S<4 leaf |
| Pennycress | S<10cm | S<10cm |
| Pansy | T | T |
| Poppy | S<10cm | Rosette |
| Red dead nettle | S<18cm | before flowering |
| Redshank | S<3 | leaf S<3 leaf |
| Scarlet pimpernel | MS | S<flowering |
| Shepherds purse | S<10cm | before flowering |
| Shepherds needle | MS | TBC |
| Speedwell, common field | T | S<4 leaf, MS>4 leaf |
| Speedwell, ivy leaved | MS<2 leaf | S<4 leaf, MS>4 leaf |
| Spurrey | – | S<2 leaf |
| Wild radish | T | T |
| Vol beans | S<8 leaf | before flowering |
| Vol borage | S<2 leaf | |
| Vol linseed | S<10cm | |
| Vol oilseed rape | T | T |
| Vol peas | S<8 leaf | before flowering |
| Vol potatoes | T | MS<20cm |
Key: bold – label weeds, S – susceptible, MS – moderately susceptible, T – tolerant; non-label weeds listed as an indication of the effect that would be expected to be achieved based on limited data.
Extensive Study
“Following the discovery of aminopyralid in 1998, an extensive study of the chemistry uncovered this group of synthetic auxins. They showed strong herbicidal activity across a range of weed species, but initially couldn’t be commercialised – there was a lack of crop selectivity and they lasted in the soil for too long. They were also fairly challenging to synthesise.”
But no one else was working in this area, and the strong herbicidal activity was enough to persuade the team to persist in their search for a compound that would meet the criteria. “Finally in 2005, we designed and made a small number of analogues with a chemical structure that was probably suitable, and one of these was Arylex.
“We put it into greenhouse tests and found it had a very broad spectrum of activity at remarkably low rates – that’s unusual for a synthetic auxin. What’s more, its soil and aqueous degradation turned out to be quite rapid. From the moment the greenhouse test results started coming in, we knew we had something that was of great interest.”
But how would this new discovery work in cold conditions? Norbert Satchivi is a senior researcher with a background in field science who joined the team in 2006 to put Arylex through its paces. “We tested the molecule in a range of different environmental conditions, but we were most interested in performance at low temperatures. The activity was particularly good in a cooler environment and that was a pleasant surprise.”
There were clearly some unique attributes of this new chemical family, so Dow worked with the University of Warwick to pinpoint why it worked at such low doses and cool temperatures.
“The target for auxins is a small family of receptor proteins,” explains Prof Richard Napier, who led the research. “Natural auxin binds to these receptors with differing affinities. Synthetic auxins also bind to them, activating a response, and this over reaction leads to plant death.
“What we found with Arylex is that it binds very tightly to some of the receptor receptor sites, more tightly than natural auxin. The way it binds with a high affinity accounts for the low dose rate and may also contribute to its efficacy at low temperatures.”
Eileen Paterson of Dow AgroSciences UK also carried out evaluations of Arylex as part of the regulatory approvals process. “We knew its activity was good, but what’s different about Arylex is its performance in cold conditions. Throughout Nov, Dec and Jan, when soil temperatures dip to just 1-2°C, you still get activity. We tested at different growth stages and timings, and the results were consistent – the activity continues when the temperature drops.”
Its weed spectrum is also considerably greater than Starane’s, she notes (see table on pxx). “It’s excellent on cleavers, but also poppies, and a broad range of common broadleaf weeds. It doesn’t control brassicas, so will need mixing with another active for volunteer oilseed rape or charlock.”
Metabolic handle
But the regulatory process has been relatively straightforward, she says. “Arylex has a unique chemical structure. In spite of its efficacy on weeds, it has a metabolic handle that’s broken down quite easily, and this means it has a good environmental profile and it also has low toxicity.
“It was the first new active ingredient that was taken through the new EU zonal regulatory process, with the UK acting as the leading authority, so it was quite an achievement,” she adds.
It also has a very good resistance profile, confirms Norbert Satchivi. “It’s a synthetic auxin herbicide, so sits in the Group O HRAC classification. Auxins work on a number of receptors in the plant, and some of these are key to processing herbicides. Because of this, it’s very difficult for a plant to evolve resistance to them. By contrast, ALS inhibitors work on a single site, and resistance can develop rapidly,” he explains.
“But although it’s in the same chemical family as other auxins, Arylex is a big departure and considerably different from other picolinates. It’s the first member of a new structural class – the arylpicolinates.”
Following on from Arylex, Rinskor is the second herbicide to be developed in this class, destined primarily for use in rice crops. And far from being outdated, this branch of chemistry has a lot to offer, he assures.
“It’s a very exciting and rewarding area of chemistry to be working in because we’re getting remarkable results from such low doses, which is unusual for auxins. This area of discovery is still on-going at Dow AgroSciences and we look forward to delivering more innovative solutions in the future.”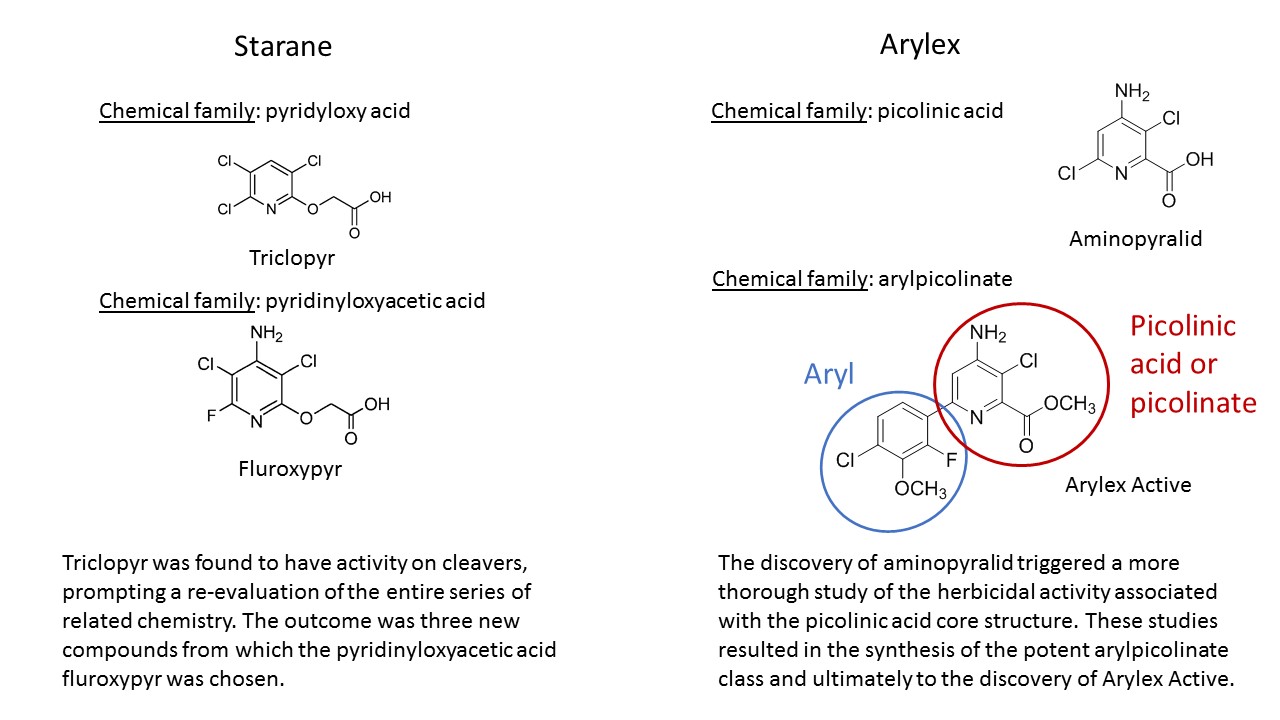
Take out the broadleaf weed worry
The difference with Arylex is that it puts the grower back in control in terms of when you spray, says Dow AgroSciences’ cereals herbicide specialist Stuart Jackson. “With Starane, you have to wait for warm conditions, but with Arylex you can apply it as soon as you can travel in the spring.”
That means broadleaf weed control can be taken care of at the T0 timing, rather than having to make a separate pass or adding it to the T1 mix, that could already have five or six products in the tank. “So it gets one job out of the way early on in the spring and allows you to focus on disease control,” he notes.
What’s more, there are few tank-mix restrictions or known antagonisms – Arylex is compatible with ALS graminicides, and there are none of the tank-mix and sequencing limitations to worry about that come with sulfonylurea (SU) herbicides, he adds.
“As well as flexibility at the front, there are no restrictions at the back of the season. There’s no risk to a following oilseed rape or sugar beet crop that you may have with an SU.
“And it essentially doesn’t matter what the weather does – Arylex will do what you want it to,” he assures. The only provisos are that the leaf should be dry at the time of application and it stays dry for an hour.
Pixxaro is currently the only Arylex product approved in the UK and can be used on winter cereals from 1 Feb or spring cereals from 1 March, and applied up to GS45. “We’re hoping for approval on the Arylex plus florasulam product in time for use this autumn. This will have an application window from 1 Sept to 30 June. The only cereal without a label use is oats, but we’re hoping to gain approval here for spring 2017.”
Priced similarly to an SU plus fluroxypyr mix, Pixxaro will have its best fit in the early spring where autumn weed control may have been compromised, advises Stuart Jackson. “If the weather closes in before the heavy autumn

Stuart Jackson highlights the few tank-mix restrictions, known antagonisms and none of the tank-mix and sequencing limitations you get with sulfonylureas.
herbicide stack goes on, you could be facing a high weed burden that’ll be too much for residuals in early spring. I can see it working well for those drilling late for blackgrass or following maize. It also fits nicely where annual meadowgrass is the main driver and you haven’t managed to get on in autumn.”
A full label dose is 0.5 l/ha, which can be cut to 0.375 l/ha if partnered with an SU, for speedwells, pansies or mayweed, for example. Only one dose of product can be applied per crop, but more than one product containing Arylex can be applied in a season.
Innovation Insight
CPM would like to thank Dow AgroSciences for kindly sponsoring this article, and for providing privileged access to staff and material used to help put the article together.




