The importance of water quality has come into sharp focus over the past few years as growers grapple with problem weeds. CPM investigates the influence of water hardness.
The pH of water may be too high to fully support the herbicide.
By Rob Jones
According to Tom Robinson, independent consultant best known to growers for his training courses on pesticide application, there’s been an awakening among growers. “They’ve become more aware of what water conditioners can do to promote spray quality and pesticide performance,” he says.
“I’m seeing a growing interest in water quality and its importance to product performance. Many products need a water conditioner to optimise performance and growers are keen to understand how they can be of benefit,” he explains.
With grassweeds becoming increasingly difficult to control, farmers are looking for incremental gains wherever they can be found. And making sure some of the herbicide isn’t being locked up by salts in the spray water is one way of getting a performance gain, he points out. But an important part of maximising the benefit from a water conditioner involves understanding how it works, so the correct mixing procedure is followed.
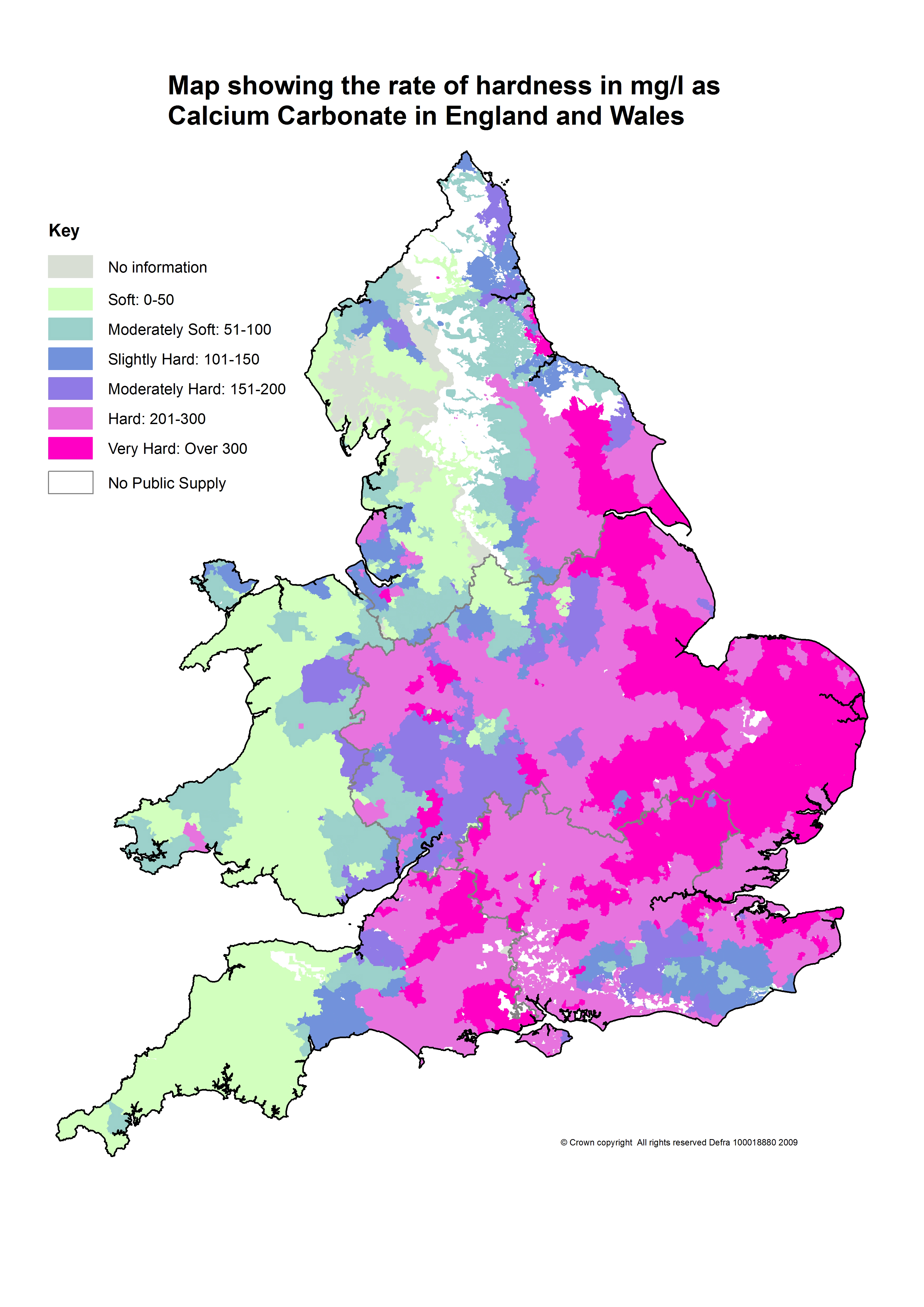
Hard water can severely affect pesticide performance. It’s an issue across England but is worst in the arable heartland of the East.
For many growers, water conditioning is the last piece in the jigsaw to better grassweed control, having already implemented delayed autumn drilling and expanded the area of spring crops. After working hard to improve seedbed quality to aid the efficacy of residual herbicides, they’ll also have considered nozzle choice and sprayer set-up, says Tom.
“When you consider that uncorrected, hard water can reduce the efficacy of certain herbicides by up to 30%, it’s easy to see where this interest is coming from,” says Rhodri Morris, commercial manager for De Sangosse.
Water is the primary carrier for applying crop protection products and constitutes more than 95% of the spray volume. Water quality can be affected by both hardness and pH, but the effects are quite distinct.
“Most growers know they should probably include a water conditioner, especially with glyphosate or sulfonylureas, but few could tell you the difference between a cation-complexing agent, an acidification agent or a competitive-salting product,” he comments.
And herein lies the issue, believes Rhodri. “There are many ways to condition water, but choosing the right method largely depends on the issue that has to be corrected. Is it a hard water issue or are you dealing with a high pH? Or does the product being applied need something more, such as a humectant to support leaf penetration or an anti-foaming agent to aid mixing?”
For most of the UK, hard water is the primary issue (see map). Reservoir and ground water nearly always have something dissolved in them, such as carbonates, bicarbonates, sulphates or nitrates. These affect the pH, taste and hardness. Glyphosate is a prime example of where performance can be impaired by hard water, says Rhodri Morris.
“Glyphosate is highly soluble, which is why it mixes so well. But it’s also an excellent chelating agent, which is where the chemical compounds react with metal ions to form a stable, water soluble complex. When added to hard water, the chelates in glyphosate bind with the cations to become locked up which makes them unavailable,” he says.
With most herbicides, the solution is to add a cation-complexing agent (such as X-Change) and this is the recommended solution from the manufacturer of Roundup (glyphosate) because it also reduces the pH, contains an anti-foamer and a humectant. But there’s a second option and that’s ammonium sulphate.
“It works differently to X-Change by out-competing the dissolved magnesium or calcium salts in the water to bind with the glyphosate, meaning it can’t be bound to anything else. The ammonium also promotes photosynthesis, which hastens the systemic activity of the herbicide,” adds Rhodri.
“While it’s better than nothing, ammonium sulphate does have its limitations as it’ll never out-compete all the dissolved salts –a complexing agent is required for this. Ammonium sulphate also does nothing to correct pH,” he says.
The pH of the water is an entirely different issue, but one that needs consideration to maximise herbicide performance, he continues. “In some situations, the pH of water may be too high to fully support the herbicide, because drinking water regulations specify that water at the tap should be between pH 6.5-9.5. In most cases, water leaving a treatment works is between pH 7-8, but it can change as it passes through the network of reservoirs and pipes.”
Even at the lowest permissible pH for tap water of pH 6.5, it’s still too high for most post-emergence herbicides. Many are classified as weak acids, so work best at a pH in the range pH 3-6, explains Rhodri.
“Above pH 7, certain pesticides can be affected by a process known as alkaline hydrolysis and this renders them permanently inactive,” he says.
“It’s a gradual process and the severity depends on several influencing factors in addition to pH, including water temperature, the amount of time elapsed after mixing and the susceptibility of the pesticide being applied. Carbamates, such as phenmedipham, and some pyrethroids, such as cypermethrin, are particularly susceptible,” he says.
“Dimethoate, an insecticide for controlling aphids in cereals and ornamentals for example, if mixed with alkaline substances (pH 9+) has a half-life of about 45 minutes. To address this issue alone, an acidifier such as Spraymac, should be considered, but this will only correct the pH of the water, while a cation complexing product will correct hard water and pH.
Is your mixing sequence right?
Conditioning the water before adding the herbicide, pesticide or insecticide is vital since the effects of hard water or high pH can’t be undone. If the appropriate mixing sequence isn’t followed, then there’s little point spending money on a water conditioner, says Tom.
The best solution, and a viable option for farms frequently using cation-complexing water conditioners, is to install an inline dosing pump to pre-treat the water entering the sprayer’s water supply tank, he suggests.
In most situations, where pre-treating the water is not an option, the key to optimising the effectiveness of the water conditioner is in the mixing sequence.
“Adding ammonium sulphate to more than a quarter tank full of water leaves it with too much competition to be very effective. As a competing agent, it can’t outcompete all the salts,” he says. “In contrast, a cation-complexer isolates the dissolved salts so they never come into contact, meaning almost all the herbicide active ingredient is available.”
To promote understanding of the effects hard water and high pH can have on pesticide performance, De Sangosse has launched ‘H₂knOw’, an initiative that seeks to help growers and agronomists to identify the specific issue with their water quality and address them correctly.
“Water conditioning is not about promoting reduced rates of use. For some products, certain sulfonylureas for example, there’s only a single rate of use and no one would suggest reducing it. Rather, water conditioning is about promoting maximum efficacy,” says Rhodri.
Criteria affecting product performance through water hardness
- Rate of product used, i.e. 1 l/ha versus 6 l/ha.
- Degree of water hardness. Ask your agronomy provider for a De Sangosse water testing kit for accurate diagnosis.
- Applied water rate/ha.
Points to consider before conditioning water
- Have you followed the correct process for testing both water hardness and pH?
- How will the result affect pesticide performance if not addressed correctly?
- What is the difference between water conditioners and salt competing agents and which is most suitable for the problem identified/product being applied?
- Are you following the correct mixing sequence for the water conditioner being used?




